Testing of CBD-infused products conducted by the U.S. Food and Drug Administration found that many are mislabeled, containing far less or more CBD than advertised and in some instances also containing THC that wasn’t supposed to be there.
102 products were tested as part of the study. Of those that were labeled as having CBD, 18 had less than 80% of the indicated amount, while 46 were within 20% of the advertised amount. Thirty-eight products had more than 120% of the CBD indicated
The results were included in an undated letter to Congress that Hemp Industry Daily obtained Wednesday. An FDA spokesman confirmed the letter but declined to comment on the findings.
The FDA tested 147 products in all. Of the 147 products, 72 contained some THC, although the concentrations were low. Products tested, included:
1. Oils
 CBD Oil is all the rage.
CBD Oil is all the rage. 2. Capsules

3. Edibles

4. Drinks
 Photo courtesy of Highsnobiety
Photo courtesy of Highsnobiety5. Pet Products
 Source: Pet Releaf
Source: Pet ReleafThe products were chosen at random after the FDA searched the internet for products. The agency also chose products from:
firms that have previously received FDA warning letters for making health claims about CBD.
industry event participants.
advertisers in trade journals.
The report repeatedly noted that the testing was from a limited sample size and that no definitive conclusions could be drawn, but “supports the need for” long-term study.
Congress weighs in
According to Hemp Industry Daily, the FDA’s report comes the same week that House budget writers called for the FDA to do more to regulate the CBD marketplace.
A subcommittee of the House Appropriations Committee set aside $5 million for the FDA to continue its review of over-the-counter CBD in the 2020-21 budget year.
In an explanatory note, the budget writers said they are concerned that CBD products “continue to pose potential health and safety risks to consumers through unsubstantiated and misleading claims such as treating a wide-range of life threatening diseases and conditions.”
Lawmakers concluded that the FDA should “continue to prioritize consumer-safety through application of the law.”
The $5 million budget for additionsal CBD review by the FDA is not final until the full Congress approves it.
Are you still missing out on The Bluntness newsletter? Sign Up today to stay in the loop.





 Cannabis and Aging: A Groundbreaking Study Challenges Long-Held Beliefs
Photo by
Cannabis and Aging: A Groundbreaking Study Challenges Long-Held Beliefs
Photo by  Cannabis and Aging: A Groundbreaking Study Challenges Long-Held Beliefs
Photo by
Cannabis and Aging: A Groundbreaking Study Challenges Long-Held Beliefs
Photo by 












 How Long Do Shrooms Last? Magic Mushroom Guide for Beginners - The Bluntness
How Long Do Shrooms Last? Magic Mushroom Guide for Beginners - The Bluntness Psilocybin can provide a life-altering experience. -The Bluntness
null
Psilocybin can provide a life-altering experience. -The Bluntness
null
 “Don’t diddle the dose. Once you have done your homework, go for it.” -- Terence McKenna
The Bluntness
“Don’t diddle the dose. Once you have done your homework, go for it.” -- Terence McKenna
The Bluntness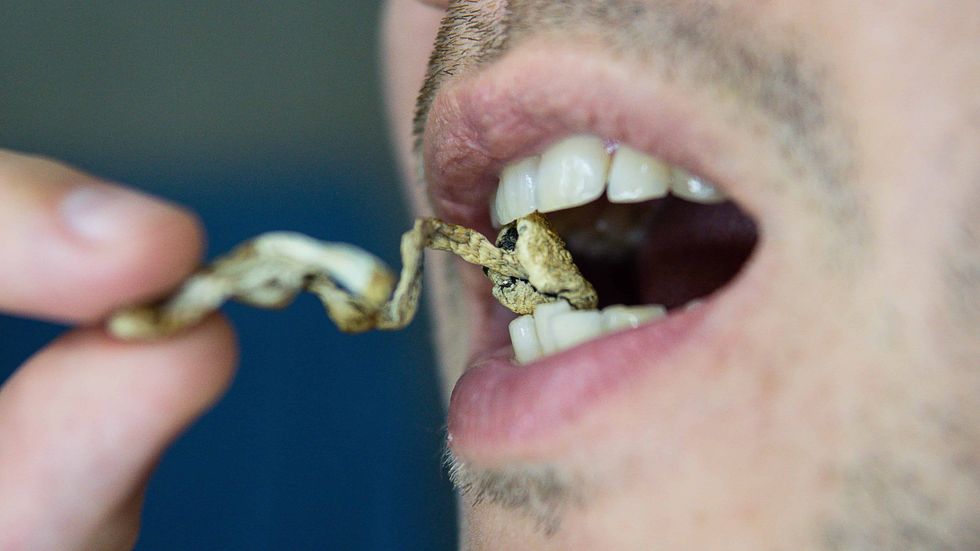 These mushrooms taste gross, but there are ways around that.The Bluntness
These mushrooms taste gross, but there are ways around that.The Bluntness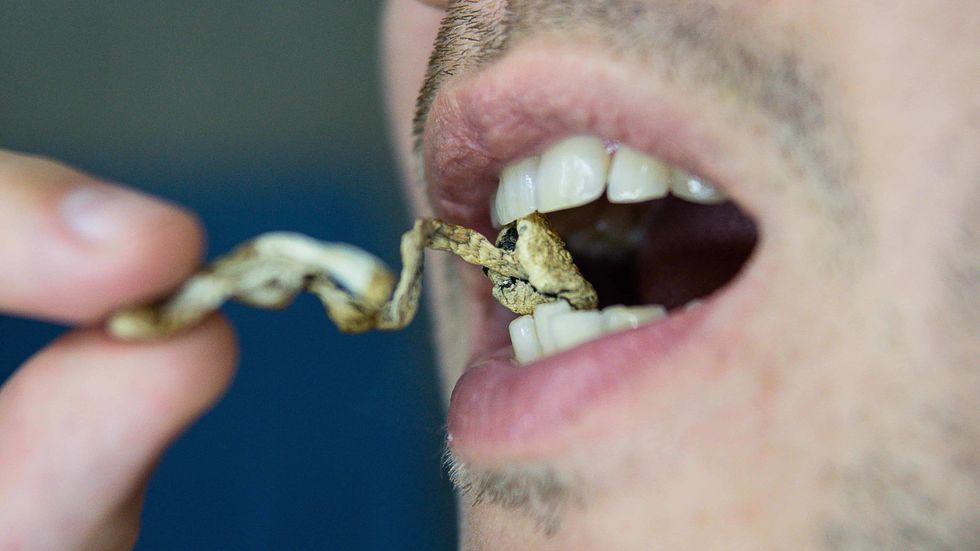 These mushrooms taste gross, but there are ways around that.
These mushrooms taste gross, but there are ways around that.
 Introduction to Psychoactive Mushrooms: The Aztec God Strain - The Bluntness
Photo by
Introduction to Psychoactive Mushrooms: The Aztec God Strain - The Bluntness
Photo by  Introduction to Psychoactive Mushrooms: The Aztec God Strain - The Bluntness
Photo by
Introduction to Psychoactive Mushrooms: The Aztec God Strain - The Bluntness
Photo by 
 FDA Approves Landmark Cannabis for PTSD in Veterans - The Bluntness
Photo by Wesley Tingey on Unsplash
FDA Approves Landmark Cannabis for PTSD in Veterans - The Bluntness
Photo by Wesley Tingey on Unsplash